Medical Services Category
Drugs for Neglected Diseases initiative (DNDi)
 The Drugs for Neglected Diseases initiative (DNDi) was established in 2003 as a not-for-profit organization which develops safe, effective and affordable drugs and treatments for people afflicted by neglected diseases.
The Drugs for Neglected Diseases initiative (DNDi) was established in 2003 as a not-for-profit organization which develops safe, effective and affordable drugs and treatments for people afflicted by neglected diseases.
Reasons for Award
There are so called twenty-one “neglected tropical diseases” that infect and afflict more than one billion people worldwide, but medicines that can treat them safely, effectively, and affordably have not been developed and manufactured due to market failures. To address this critical challenge with humanity and bravery, DNDi was founded in 2003 with the contribution of a portion of the Nobel Peace Prize awarded to the Médecins Sans Frontières (MSF). Since its inception, DNDi, along with its international partners, has developed and delivered 13 affordable new treatments for six deadly diseases, including African sleeping sickness, visceral leishmaniasis, pediatric HIV, and malaria, with broad impact in improving global health, particularly in low- and middle-income countries. Among these, fexinidazole, the first oral treatment developed by DNDi with its partners for the treatment of Gambian-type human African trypanosomiasis (gHAT, a form of African sleeping sickness), which is severely devastating in sub-Saharan Africa, has brought about a breakthrough effect in the endemic countries. In addition, through the HAT Platform, a network of research institutions and experts mainly from endemic countries in Africa, DNDi has helped overcome significant challenges such as conducting clinical studies in remote areas in accordance with international scientific quality standards. Based on these achievements, DNDi and its partners are working continuously toward the final target of the elimination of sleeping sickness.
Summary of Achievements
The Drugs for Neglected Diseases initiative (DNDi) was created in response to the frustration of clinicians and the desperation of patients faced with medicines that were ineffective, unsafe, unavailable, unaffordable, or that had never been developed at all. The Kenyan Medical Research Institute (KEMRI), the Indian Council of Medical Research (ICMR), the Oswaldo Cruz Foundation in Brazil, the Malaysian Ministry of Health, and the Institut Pasteur of France, with the participation of World Health Organization Special Programme on Research and Training in Tropical Diseases (WHO/TDR), teamed up with MSF to found this organization.
DNDi prioritizes the needs of vulnerable and neglected people, including women and children, in low and middle-income countries, and develops and delivers new treatments for sleeping sickness, leishmaniasis, Chagas disease, river blindness, mycetoma, dengue, pediatric HIV, advanced HIV disease, cryptococcal meningitis, and hepatitis C. Since its inception in 2003, DNDi and its partners have delivered 13 affordable new treatments for six deadly diseases, out of which, 9 new treatments have been developed and delivered in Africa for four deadly diseases: African sleeping sickness (human African trypanosomiasis:HAT), visceral leishmaniasis, pediatric HIV, and malaria. DNDi estimates that neglected patients have benefited from at least 542 million treatments developed and delivered by DNDi and its partners since 2007.
One remarkable example of DNDi’s achievement is the development and delivery of fexinidazole-the game-changing drug for T.b.gambiense sleeping sickness (gHAT) that killed hundreds of thousands of African people in the past century. People affected by this disease are some of the most vulnerable and live in some of the most remote and conflict-affected areas. For decades, its treatment was complex, difficult to administer, and even toxic. DNDi and its partners made continuous efforts, and developed and delivered fexinidazole, the first all-oral treatment for gHAT. It is an oral pill taken for 10 days, offering practical advantages over the previous standard of care, because it removes the need for systematic hospitalization and eases the burden on health systems, and leads to a reduction in the number of painful lumbar punctures for staging.
Fexinidazole was registered in the Democratic Republic of Congo in December 2018, where over 60% of sleeping sickness cases have been reported, and in Uganda in October 2021. In June 2019, fexinidazole was added to the World Health Organization (WHO) Essential Medicines Lists for children and adults. In August 2019, WHO published new sleeping sickness treatments guidelines to include fexinidazole as the first-line treatment for gHAT patients. All gHAT endemic countries are now utilizing fexinidazole as a first-line treatment.
DNDi has been instrumental in making fexinidazole available to gHAT patients, partnering with Sanofi, WHO, the HAT Platform and national control programs in endemic countries. Since 2005, DNDi has been supporting the creation and interactions of the HAT Platform, a network of 120 experts from more than 20 research institutions in endemic countries. DNDi’s close collaboration with national sleeping sickness control programs and the HAT Platform helped overcome the significant challenges to conducting clinical research in very remote settings in accordance with international ethical and scientific quality standards, facilitate access to and uptake of new treatments and advocate for an enabling policy and regulatory environment to meet the needs of the most neglected in endemic countries.

Dr Patou, a doctor from the national control programme for sleeping sickness in the Democratic Republic of Congo, hands over a box of fexinidazole to the boat captain ready for their journey upstream on the river Kwilu until Mushie. Dr Patou and his team are bringing the new treatment to the remote health post there as new patients have been detected. (Photo Credit : Kenny Mbala-DNDi)
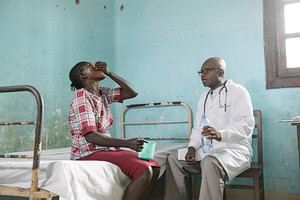
Antoinette Mpono Bukoy receiving fexinidazole from Dr Kandé, at Masi Manimba hospital in the Democratic Republic of Congo (DRC). (Photo Credit : Xavier Vahed/DNDi)
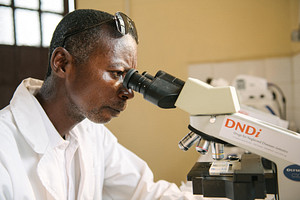
Lab technician Leon Katunda analyses cerebrospinal fluid under a microscope, as a part of DNDi’s trials for safer and more effective sleeping sickness treatments in Masi Manimba, Democratic Republic of Congo. (Photo Credit:Xavier Vahed/DNDi)
